Recombinant Rat Cytochrome b5 (Cyb5a), partial
-
中文名稱:Recombinant Rat Cytochrome b5(Cyb5a) ,partial
-
貨號:CSB-YP006309RA1
-
規(guī)格:
-
來源:Yeast
-
其他:
-
中文名稱:Recombinant Rat Cytochrome b5(Cyb5a) ,partial
-
貨號:CSB-EP006309RA1
-
規(guī)格:
-
來源:E.coli
-
其他:
-
中文名稱:Recombinant Rat Cytochrome b5(Cyb5a) ,partial
-
貨號:CSB-EP006309RA1-B
-
規(guī)格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:Recombinant Rat Cytochrome b5(Cyb5a) ,partial
-
貨號:CSB-BP006309RA1
-
規(guī)格:
-
來源:Baculovirus
-
其他:
-
中文名稱:Recombinant Rat Cytochrome b5(Cyb5a) ,partial
-
貨號:CSB-MP006309RA1
-
規(guī)格:
-
來源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:Cyb5a
-
Uniprot No.:
-
別名:Cyb5a; Cyb5; Cytochrome b5
-
種屬:Rattus norvegicus (Rat)
-
蛋白長度:Partial
-
蛋白標簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相關(guān)產(chǎn)品
靶點詳情
-
功能:Cytochrome b5 is a membrane-bound hemoprotein functioning as an electron carrier for several membrane-bound oxygenases. It is also involved in several steps of the sterol biosynthesis pathway, particularly in the C-6 double bond introduction during the C-6 desaturation.
-
基因功能參考文獻:
- Cygb has a nitric-oxide dioxygenase function and ascorbate and cytochrome b(5) have roles as reductants PMID: 20511233
- Chemical shift-based constraints for solution structure determination of cytochrome b5 PMID: 11941499
- The hydrophobic clusters contribute to the greater stability of rat outer mitochondrial membrane Cyb5 and are responsible for higher kinetic barriers of hemin release and hemin reorientation compared to bovine microsomal apocyt b5. PMID: 12269800
- outer mitochondrial membrane cytochrome b5 and not microsomal membrane cytochrome b5 functions as an activator for androgenesis in rat Leydig cells. PMID: 12668680
- b5 is integrated into the ER membrane via the loosely bound state, which is described, and the 3 amino acids, -Leu-Met-Tyr, are important for conversion of the loosely-bound state to the firmly-integrated state. PMID: 12761189
- when rat endoplasmic reticulum cytochrome b5 was coexpressed with wild-type desaturase, both proteins interacted and Delta6-desaturase activity was significantly increased PMID: 14563830
- The first mutation (D60R) of cytochrome b(5), stabilized the holoprotein in a probable manifestation of enhanced helical propensity or improved electrostatic interactions PMID: 15379561
- simulations provided qualitative microscopic explanations of many of the differences in physical properties between outer mitochondrial membrane CYB5 isoform and microsomal CYB5 and two mutants in terms of localized changes in structure and flexibility PMID: 16807901
- The data distinguished the four helical segments and provided insight into the existence of holo- and nonholo-like interactions in the cytochrome's heme binding site. PMID: 18398853
- The effect of the His63-iron bond and proximity of heme plane on the population of helical conformation in H4 and H5 of cytochrome b5 was investigated. PMID: 18398854
- The hydrophobic domain of cytochrome b5 participates not only in hemeprotein interaction, but also in electron transfer from cytochrome b5 to cytochrome P4503A4. PMID: 19817686
顯示更多
收起更多
-
亞細胞定位:Endoplasmic reticulum membrane; Single-pass membrane protein; Cytoplasmic side. Microsome membrane; Single-pass membrane protein; Cytoplasmic side.
-
蛋白家族:Cytochrome b5 family
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
Recombinant Human Mesothelin (MSLN), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
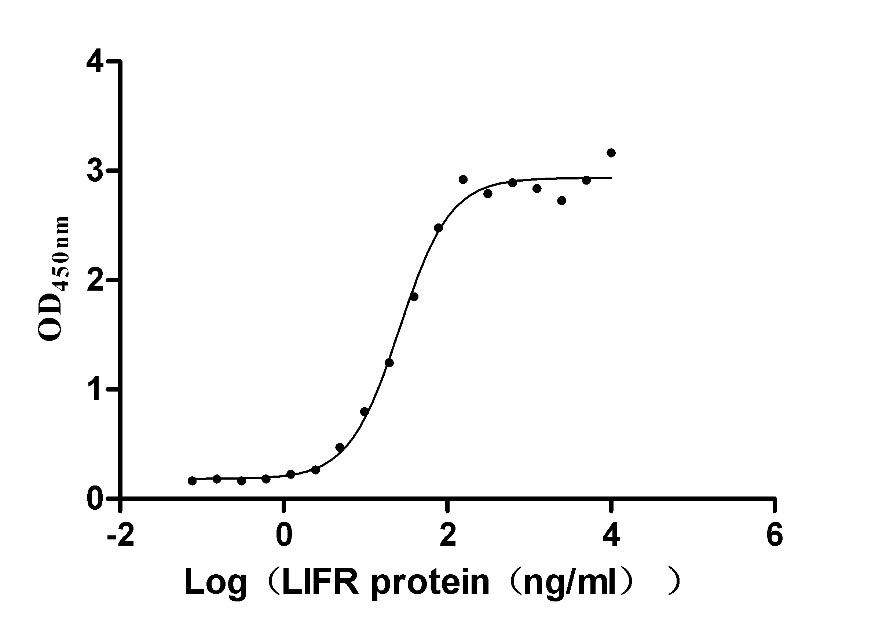
Recombinant Human Leukemia inhibitory factor (LIF) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
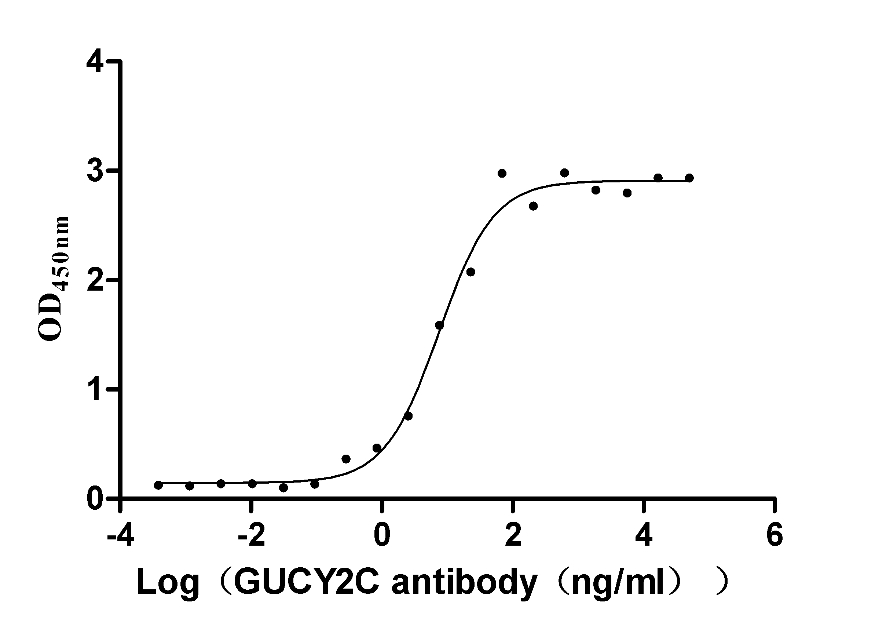
Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
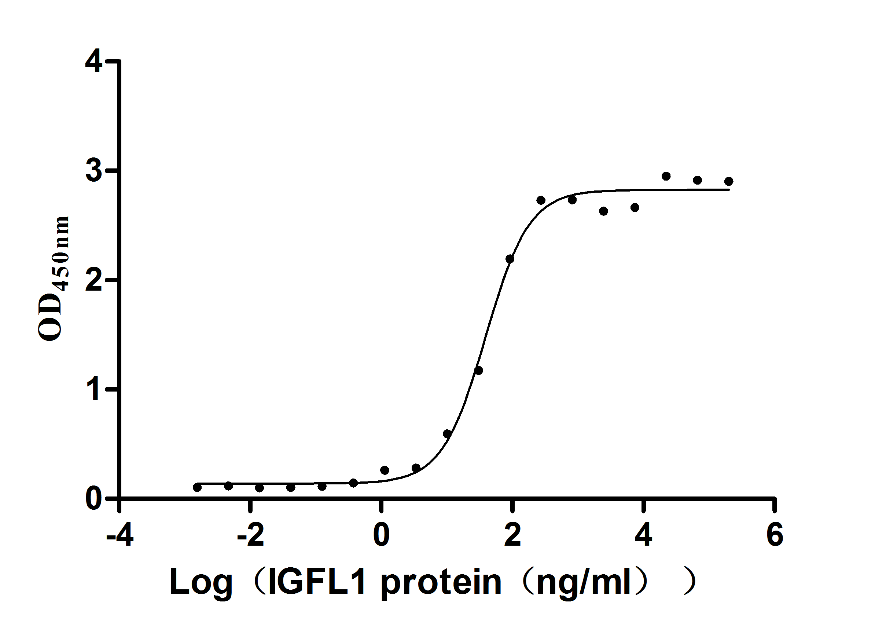
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
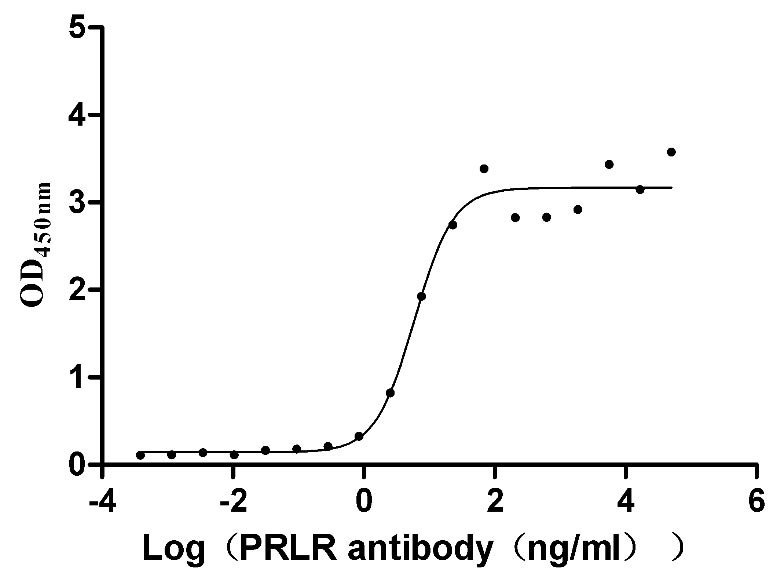
Recombinant Mouse Prolactin receptor (Prlr), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Claudin-18.2 (CLDN18.2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Intestinal-type alkaline phosphatase (ALPI) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Rat Intestinal-type alkaline phosphatase 1 (Alpi) (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)



-AC1.jpg)












